Human Physiology
Cardiovascular system
The Cardiovascular System:
- consists of the heart plus all the blood vessels
- transports blood to all parts of the body in two 'circulations': pulmonary (lungs) & systemic (the rest of the body)

- hollow, muscular organ
- 4 chambers: 2 atria (right & left) & 2 ventricles (right & left)
Cardiovascular system
Heart anatomy and function
Path of a red blood cell
- 1 - endocardium - innermost layer; epithelial tissue that lines the entire circulatory system 2 - myocardium - thickest layer; consists of cardiac muscle
3 - epicardium - thin, external membrane around the heart

- striated (see photo below; consists of sarcomeres just like skeletal muscle)
- cells contain numerous mitochondria (up to 40% of cell volume)
- adjacent cells join end-to-end at structures called intercalated discs

- desmosomes (which act like rivets & hold the cells tightly together) and
- gap junctions (which permit action potentials to easily spread from one cardiac muscle cell to adjacent cells).

- the atria being one &
- the ventricles the other.
- contractile cells &
- autorhythmic (or automatic) cells.


On the left is the action potential of an autorhythmic cell; on the right, the action potential of a contractile cell.
- the slow calcium channels open (4),
- then concludes (quickly) when the fast calcium channels open (0).
- Repolarization is due to the outward diffusion of potassium (3).

Used with permission: http://mail.bris.ac.uk/~pydml/CVS/Heart/Cells/Electrics/APpmr.htm
- depolarization is very rapid & is due to the inward diffusion of sodium (0).
- repolarization begins with a slow outward diffusion of potassium, but that is largely offset by the slow inward diffusion of calcium (1 & 2). So, repolarization begins with a plateau phase. Then, potassium diffuses out much more rapidly as the calcium channels close (3), and the membrane potential quickly reaches the 'resting' potential (4).

Used with permission: http://mail.bris.ac.uk/~pydml/CVS/Heart/Cells/Electrics/APpmr.htm
Most of the muscle cells in the heart are contractile cells. The autorhythmic cells are located in these areas:
- Sinoatrial (SA), or sinus, node

- Atrioventricular (AV) node
- Atrioventricular (AV) bundle (also sometimes called the bundle of His)
- Right & left bundle branches
- Purkinje fibers
AV node & AV bundle - 40 - 60 per minute
Bundle branches & Purkinje fibers - 20 - 40 per minute
SA node = has the highest or fastest rhythm &, therefore, sets the pace or rate of contraction for the entire heart. As a result, the SA node is commonly referred to as the PACEMAKER.
Spread of cardiac excitation (check this animation: Conducting System of the Heart):
- Begins at the SA node & quickly spreads through both atria
- Also travels through the heart's 'conducting system' (AV node > AV bundle > bundle branches > Purkinje fibers) through the ventricles
- For efficient pumping:
- The atria should contract (& finish contracting) before the ventricles contract. This occurs because of AV nodal delay (that is, the impulse travels rather slowly through the AV node & this permits the atria to complete contraction before the ventricles begin contraction).
- The atria should contract as a unit, & the ventricles should contract as a unit. This occurs because the impulse spreads so rapidly that all myocardial cells in the atria and ventricles, respectively, contract at about the same time. The impulse spreads rapidly through the ventricles because of the conducting system.

Refractory period of contractile cells:
- Lasts about 250 msec (almost as long as contraction period)

 Electrocardiogram (ECG) = record of spread of electrical activity through the heart
Electrocardiogram (ECG) = record of spread of electrical activity through the heart P wave = caused by atrial depolarization
QRS complex = caused by ventricular depolarization
T wave = caused by ventricular repolarization
ECG = useful in diagnosing abnormal heart rates, arrhythmias, & damage of heart muscle
Electrocardiogram
 | Coronary artery disease (CAD) is a condition in which plaque builds up inside the coronary arteries that supply heart muscle with oxygen-rich blood. Plaque is made up of fat, cholesterol, calcium, and other substances found in the blood. When plaque builds up in the arteries, the condition is called atherosclerosis. Plaque narrows the arteries and reduces blood flow to your heart muscle. It also makes it more likely that blood clots will form and partially or completely block blood flow. When coronary arteries are narrowed or blocked, oxygen-rich blood can't reach the heart muscle. This can cause angina or a heart attack. Angina is chest pain or discomfort that occurs when not enough blood flows to an area of heart muscle. A heart attack occurs when blood flow to an area of heart muscle is completely blocked. This prevents oxygen-rich blood from reaching that area of heart muscle and causes it to die. Without quick treatment, a heart attack can lead to serious problems and even death. Over time, CAD can weaken heart muscle and lead to heart failure and arrhythmias. Heart failure is a condition in which your heart can't pump enough blood throughout your body. Arrhythmias are problems with the speed or rhythm of your heartbeat. CAD is the most common type of heart disease. It's the leading cause of death in the United States for both men and women. Lifestyle changes, medicines, and/or medical procedures can effectively prevent or treat CAD in most people (Source: NHLBI). |
Heart Valves:
- Atrioventricular (AV) valves - prevent backflow of blood from ventricles to atria during ventricular systole (contraction)
- Tricuspid valve - located between right atrium & right ventricle
- Mitral valve - located between left atrium & left ventricle
- Semilunar valves - prevent backflow of blood from arteries (pulmonary artery & the aorta) to ventricles during ventricular diastole (relaxation)
- Aortic valve - located between left ventricle & the aorta
- Pulmonary valve - located between right ventricle & the pulmonary artery (trunk)
- AV valves - open when pressure in the atria is greater than pressure in the ventricles (i.e., during ventricular diastole) & closed when pressure in the ventricles is greater than pressure in the atria (i.e., during ventricular systole)
- Semilunar valves - open when pressure in the ventricles is greater than pressure in the arteries (i.e., during ventricular systole) and closed when pressure in the pulmonary trunk & aorta is greater than pressure in the ventricles (i.e., during ventricular diastole)

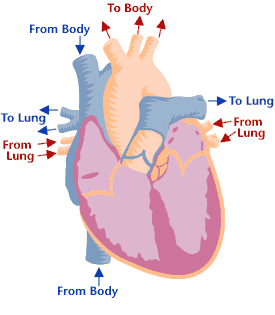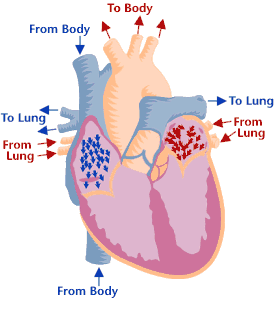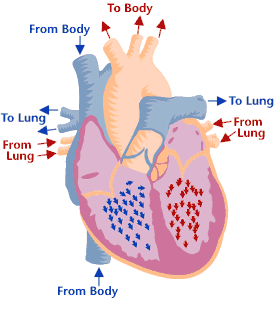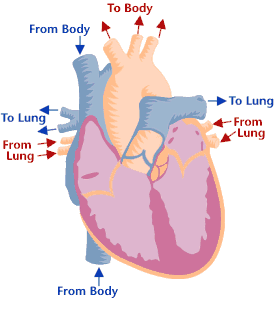
Heart valves & function
 Cross-section of a healthy heart, including the four heart valves. The blue arrow shows the direction in which oxygen-poor blood flows from the body to the lungs. The red arrow shows the direction in which oxygen-rich blood flows from the lungs to the rest of the body. Cross-section of a healthy heart, including the four heart valves. The blue arrow shows the direction in which oxygen-poor blood flows from the body to the lungs. The red arrow shows the direction in which oxygen-rich blood flows from the lungs to the rest of the body. | Heart valve disease is a condition in which one or more heart valves don't work properly, making the heart work harder and affecting its ability to pump blood. Malfunctioning heart valves can create two basic problems: (1) Regurgitation, or backflow, occurs when a valve doesn’t close tightly. Blood leaks back into the chamber rather than flowing forward through the heart or into an artery. Backflow is most often due to prolapse (the flaps of the valve flop or bulge back into an upper heart chamber during a heartbeat). (2) Stenosis occurs when the flaps of a valve thicken, stiffen, or fuse together. This prevents the heart valve from fully opening, and not enough blood flows through the valve. You can be born with heart valve disease (congenital) or you can acquire it later in life. Although a valve may be normal at first, disease can cause problems to develop over time. Many people have heart valve defects or disease, but don't have symptoms. For some people, the condition will stay largely the same over their lifetime and not cause any problems. For other people, the condition can worsen slowly over time until symptoms develop. If not treated, advanced heart valve disease can cause heart failure, stroke, blood clots, or sudden death due to cardiac arrest. Lifestyle changes and medicines can relieve many of the symptoms and problems linked to heart valve disease, and can also lower the risk of developing a life-threatening condition, such as stroke or sudden cardiac arrest. Eventually, however, faulty heart valves may have to be repaired or replaced (Source: NHLBI). |
Mechanical Events of the Cardiac Cycle: (also check www-medlib.med.utah.edu/kw/pharm/hyper_heart1.html and McGraw-Hill.com)
- the cardiac cycle has two phases: systole (contraction) & diastole (relaxation)
- 'Electrical' events are correlated with the 'mechanical' events:
- P wave = atrial depolarization = atrial systole
- QRS complex = ventricular depolarization = ventricular systole (& atrial diastole occurs at the same time)
- T wave = ventricular repolarization = ventricular diastole
- What happens in the heart during each 'mechanical' event:
- Atrial systole (labeled AC below):
- no heart sounds (because no heart valves are opening or closing)
- a slight increase in ventricular volume because blood from the atria is pumped into the ventricles
- Ventricular systole:
- the first heart sound (lub) (labeled S1 below) - this sound is generated by the closing of the AV valves (& this occurs because increasing pressure in the ventricles causes the AV valves to close)
- initially there is no change in ventricular volume (called the period of isometric contraction) because ventricular pressure must build to a certain level before the semilunar valves can be forced open & blood ejected. Once that pressure is achieved, & the semilunar valves do open, ventricular volume drops rapidly as blood is ejected.
- Ventricular diastole:
- the second heart sound (dub) (labeled S2 below) - this sound is generated by the closing of the semilunar valves (& this occurs because pressure in the pulmonary trunk & aorta is now greater than in the ventricles & blood in those vessels moves back toward the area of lower pressure which closes the valves)
- ventricular volume increases rapidly (period of rapid inflow) - this occurs because blood that accumulated in the atria during ventricular systole (when the AV valves were closed) now forces open the AV valves (because the pressure in the atria is now greater than the pressure in the ventricles). & flows quickly into the ventricles. After this 'rapid inflow', ventricular volume continues to increase, but at a slower rate (the period of diastasis). This increase in volume occurs as blood returning to the heart via the veins largely flows through the atria & into the ventricles.
 Used with permission: http://mail.bris.ac.uk/~pydml/CVS/Heart/Whole/CardCyc/CCprvo.htm
Used with permission: http://mail.bris.ac.uk/~pydml/CVS/Heart/Whole/CardCyc/CCprvo.htm 


Human heart
Cardiac output:
- volume of blood pumped by each ventricle
- equals heart rate (beats per minute) times stroke volume (milliliters of blood pumped per beat)
- typically about 5,500 milliliters (or 5.5 liters) per minute (which is about equal to total blood volume; so, each ventricle pumps the equivalent of total blood volume each minute under resting conditions) BUT maximum may be as high as 25 - 35 liters per minute
- the difference between cardiac output at rest & the maximum volume of blood the heart is capable of pumping per minute
- permits cardiac output to increase dramatically during periods of physical activity
What factors permit variation in cardiac output?
- Changes in heart rate:
- Parasympathetic stimulation - reduces heart rate
- Sympathetic stimulation - increases heart rate
Effect of parasympathetic stimulation on the heart:
Increased parasympathetic stimulation > release of acetylcholine at the SA node > increased permeability of SA node cell membranes to potassium > 'hyperpolarized' membrane > fewer action potentials (and, therefore, fewer contractions) per minute

a = sympathetic stimulation, b = normal heart rate, & c = parasympathetic stimulation
Effect of sympathetic stimulation on the heart:
Increased sympathetic stimulation > release of norepinephrine at SA node > decreased permeability of SA node cell membranes to potassium > membrane potential becomes less negative (closer to threshold) > more action potentials (and more contractions) per minute
Regulation of Stroke Volume:
- intrinsic control ==> related to amount of venous return (amount of blood returning to the heart through the veins)
- extrinsic control ==> related to amount of sympathetic stimulation
Intrinsic control:
- Increased end-diastolic volume = increased strength of cardiac contraction = increased stroke volume
- This increase in strength of contraction due to an increase in end-diastolic volume (the volume of blood in the heart just before the ventricles begin to contract) is called the Frank-Starling law of the heart:
- Increased end-diastolic volume = increased stretching of of cardiac muscle = increased strength of contraction = increased stroke volume

Source: http://www.sci.sdsu.edu/Faculty/Paul.Paolini/ppp/lecture21/sld006.htm
- Increased sympathetic stimulation > increased strength of contraction of cardiac muscle
- Mechanism = sympathetic stimulation > release of norepinephrine > increased permeability of muscle cell membranes to calcium > calcium diffuses in > more cross-bridges are activated > stronger contraction

Flow rate through blood vessels
- directly proportional to the pressure gradient
- inversely proportional to vascular resistance
Resistance:
- hindrance to blood flow through a vessel caused by friction between blood & vessel walls
- major determinant = vessel diameter (or radius)
- is inversely proportional to radius to the fourth power (so, for example, doubling the radius of a vessel decreases the resistance 16 times which, in turn, increases flow through the vessel 16 times)


Source: http://www.oucom.ohiou.edu/CVPhysiology/H003.htm
Arteries:
- serve as passageways for blood from heart to tissues
- act as pressure reservoirs because the elastic walls collapse inward during ventricular diastole (when there is less blood in the arteries):

- blood pressure averages 120 mm Hg during systole (systolic pressure) & 80 mm Hg during diastole (diastolic pressure) (& the difference between systolic & diastolic pressures is called the pulse pressure)
Arterioles:
- distribute cardiac output among systemic organs (whose needs vary over time)
- Resistance (&, therefore, blood flow) varies as a result of VASODILATION & VASOCONSTRICTION
- Factors that influence radius of arterioles:
- intrinsic (or local) control
- extrinsic control

- changes within a tissue that alter the radius of blood vessels & adjust blood flow
- especially important in skeletal muscles, the heart, & the brain
- increased blood flow in an active tissue results from active hyperemia:
 turn, causes vasodilation of the arterioles > vasodilation reduces resistance with the vessel &, as a result, blood flow through the vessel increases So, blood flow increases when a tissue (e.g., skeletal muscle) becomes more active & the increased blood flow delivers the needed oxygen & nutrients.
turn, causes vasodilation of the arterioles > vasodilation reduces resistance with the vessel &, as a result, blood flow through the vessel increases So, blood flow increases when a tissue (e.g., skeletal muscle) becomes more active & the increased blood flow delivers the needed oxygen & nutrients. Extrinsic control occurs via:
- sympathetic division of the Autonomic Nervous System
- parasympathetic division of the Autonomic Nervous System
Capillaries

- site of exchange of materials between blood & tissues
- exchange may occur by simple diffusion
- diffusion enhanced by:
- thin capillary walls (just one cell thick)
- narrow capillaries (so the red blood cells & plasma are close to the walls)
- large numbers (the human body has 10 - 40 billion capillaries!) which translates into a tremendous amount of surface area through which exchange can occur
- relatively slow flow of blood (providing more time for exchange to occur)
- exchange also occurs through pores (located between the cells the form the capillary walls), by vesicular transport (e.g., pinocytosis), & by bulk flow

- protein-free plasma filters out of capillaries, mixes with surrounding interstitial fluid, & is then reabsorbed. Plasma filters out at the arteriole end of capillaries because hydrostatic (blood) pressure (an outward force) exceeds osmotic pressure (an inward force). At the venous end of capillaries, the filtrate tends to move back in because osmotic pressure now exceeds hydrostatic pressure (check this animation at mcgraw-hill.com).
- because the outward force at the arteriole end exceeds the inward force at the venous end, more plasma filters out than moves back in to the capillaries. So, fluid tends to accumulate in the tissues. The lymph vessels pick up this fluid & transport it back to the blood.

- 1 - not very important in exchange (much more exchange occurs by way of diffusion) 2 - important in regulating the 'distribution' of fluids between the plasma & interstitial fluid (which is important in maintaining normal blood pressure)
Veins:
- serve as low-resistance passageways to return blood from the tissues to the heart
- serve as a BLOOD RESERVOIR (under resting conditions nearly two-thirds of all your blood in located in the veins) &, therefore, the veins are important in permitting changes in stroke volume


 The reaction of group 1 metals with water
The reaction of group 1 metals with water In aqueous solutions the metal cations formed are hydrated to aqa-complex ions
In aqueous solutions the metal cations formed are hydrated to aqa-complex ions (I will replace with proper diagrams later)
(I will replace with proper diagrams later)